
Important Takeaways:
- Robert F Kennedy Jr has pulled promotional ads for vaccines and postponed a meeting of key vaccine advisors, in one of his first moves as health secretary.
- Kennedy wants the CDC to move away from nudge tactics and focus its vaccine communications on ‘informed consent’ – which involves telling the patient the medical risks and benefits and letting them come to their own decision.
- Meanwhile, the year’s first meeting of the CDC’s influential panel of vaccine experts has been delayed indefinitely, marking the first time the meeting has been postponed in over 40 years, except for during the emergency Covid pandemic.
- RFK released his blueprint for reevaluating recommended vaccines, shifting research priorities, removing legal protections for vaccine manufacturers, and modifying vaccine advertising practices years ago.
- It calls for subjecting vaccines to the same rigorous approval process as other drugs, mandating automated adverse event reporting, eliminating conflicts of interest in federal vaccine approvals, and reevaluating all vaccines recommended before evidence-based guidelines were established.
Read the original article by clicking here.

Important Takeaways:
- Health officials have said that the overall risk to the American population is low, but the Centers for Disease Control and Prevention has confirmed 61 human cases of the bird flu since April 2024.
- On Wednesday, California governor Gavin Newsom, whose state has seen the bulk of the cases confirmed so far, issued a state of emergency in connection with the bird flu
- Although the majority of the reported cases have come with only mild symptoms, this week the agency reported a new severe case of the virus discovered in Louisiana that resulted in hospitalization for the patient
- The Louisiana Department of Health tells CNN that the patient in question is older than 65 and is currently hospitalized in critical condition with “severe respiratory illness,” but also has other underlying health conditions.
- “While an investigation into the source of the infection in Louisiana is ongoing, it has been determined that the patient had exposure to sick and dead birds in backyard flocks,” the agency said, noting that this is also the first bird-flu case to be traced back to backyard flocks.
Read the original article by clicking here.

Important Takeaways:
- An Iowa resident has died after contracting a frightening viral disease, similar to Ebola, that leaves victims bleeding from their eyeballs.
- The patient had returned to the U.S. from West Africa earlier this month bringing the disease known as Lassa Fever, rarely seen in the U.S., back with them, health officials said.
- The person was not sick while traveling meaning the risk to fellow airline passengers is ‘extremely low,’ officials with the U.S. Centers for Disease Control and Prevention said.
- Patients are not believed to be infectious before symptoms occur and the virus is not spread by casual contact.
- The patient, who has not been identified publicly, was placed in isolation in hospital at the University of Iowa Health Care Medical Center in Iowa City.
- On Monday, testing by the Nebraska Laboratory Response Network revealed the patient had died from Lassa Fever.
- If the results are confirmed, the Iowa case would be the ninth known case of Lassa Fever since 1969 in travelers returning to the U.S. from areas where the disease is found.
- The CDC is now assisting Iowa health officials to identify people who had been in contact with the patient after symptoms began. Those identified as being in close contact will be monitored for three weeks.
- Lassa Fever, which is caused by the Lassa virus, is a relatively common disease in West Africa, with between 100,000 and 300,000 cases diagnosed every year with around 5,000 deaths.
Read the original article by clicking here.

Important Takeaways:
- A deadly E. coli outbreak linked to McDonald’s Quarter Pounders has led to 75 cases in 13 states, the Centers for Disease Control and Prevention said on Friday, as it investigates the source of the spread.
- The outbreak has led to 22 hospitalizations and one previously reported death of an older adult in Colorado.
- Out of 61 patients with information available, 22 have been hospitalized and two people have developed a serious condition that can cause kidney failure, called hemolytic uremic syndrome. All of the 42 people who were interviewed by the CDC reported eating at McDonald’s, while 39 people reported eating a beef hamburger, the agency said.
- Those with infections ranged from ages 13 to 88, according to the CDC. The agency reiterated that the number of cases in the outbreak is likely much higher than what has been reported so far. The CDC added that the outbreak may not be limited to the states with related cases. That is because many patients don’t test for E. coli and recover from an infection without receiving medical care, the CDC said. It also usually takes three to four weeks to determine if a sick person is part of an outbreak.
- Those with infections ranged from ages 13 to 88, according to the CDC. The agency reiterated that the number of cases in the outbreak is likely much higher than what has been reported so far. The CDC added that the outbreak may not be limited to the states with related cases. That is because many patients don’t test for E. coli and recover from an infection without receiving medical care, the CDC said. It also usually takes three to four weeks to determine if a sick person is part of an outbreak.
- McDonald’s declined to comment on the update, citing the company’s statement when the outbreak was first announced.
Read the original article by clicking here.

Important Takeaways:
- A bird flu pandemic is inevitable – and it’s only a matter of time before it strikes, according to former Centers for Disease Control and Prevention (CDC) Director Robert Redfield.
- Redfield’s comments come amid mounting concerns over the detection of the virus in dozens of cattle herds across the United States and the first reported human death in Mexico
- In a recent interview with NewsNation, Redfield expressed his belief that a bird flu pandemic is a high likely. “I really do think it’s very likely that we will, at some time,” he said. “It’s not a question of if; it’s more of a question of when we will have a bird flu pandemic.” He emphasized the significant mortality rate associated with the virus, with an estimation of a mortality rate of “somewhere between 25 and 50 percent,” in contrast to the 0.6 percent death rate observed in the Covid-19 pandemic.
- Redfield explained that the key to the virus’s ability to spread from human to human lies in the change of five specific amino acids in a critical receptor. Once the virus acquires this capacity, the pandemic could be unleashed. Redfield stated, “That’s when you’re going to have the pandemic. And as I said, I think it’s just a matter of time.”
Read the original article by clicking here.
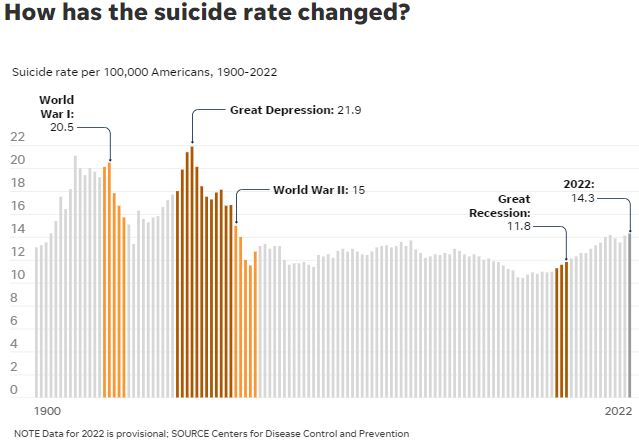
Important Takeaways:
- The suicide rate among Americans, which has risen steadily over the past 18 years, has reached its highest point since 1941, preliminary data for 2022 shows.
- The suicide rate per 100,000 people in 2022 was 14.3, according to a report from the Centers from Disease Control and Prevention released early Wednesday. The rate was 15 in 1941.
- An estimated 49,449 people died by suicide in 2022, the CDC said. That’s an increase of 2.6% over the 48,183 suicide deaths in 2021.
- The rate for males was 23.1 and 5.9 for females in 2022.
Read the original article by clicking here.

The American Heritage Dictionary “plagues”
1. A highly infectious, usually fatal, epidemic disease; a pestilence.
2. A virulent, infectious disease that is caused by the bacterium Yersinia pestis (syn. Pasteurella pestis) and is transmitted primarily by the bite of fleas from an infected rodent, especially a rat. In humans it occurs in bubonic form, marked by lymph node enlargement, and in pneumonic form, marked by infection of the lungs, and can progress to septicemia.
3. A widespread affliction or calamity seen as divine retribution.
Important Takeaways:
- Biden Administration to Urge All Americans: Get a Coronavirus Booster Shot Now
- All Americans will be urged by the Biden administration to get a coronavirus booster shot this autumn ahead of what it claims is a new wave of infections, a White House official said Sunday.
- Reuters reports the official said while the Centers for Disease Control and Prevention reports an increase in infections and hospital admissions from the virus, overall levels remain low, however caution is urged.
- Moderna and other coronavirus vaccine makers Novavax, Pfizer and German partner BioNTech SE have all reportedly created versions of their shots ready and aimed at the XBB.1.5 subvariant.
Read the original article by clicking here.

Important Takeaways:
- CDC bought cellphone data to track vaccination, lockdown compliance: report
- The Centers for Disease Control and Prevention used location data from tens of millions of Americans’ phones to track compliance with lockdown orders and vaccination efforts, according to newly revealed documents.
- The CDC specifically monitored Americans’ visits to churches and schools, as well as “detailed counts of visits to participating pharmacies for vaccine monitoring,” internal documents from the federal agency obtained by Vice show.
- The CDC also reportedly tracked peoples’ movement during curfews and visits between neighbors.
- A controversial “data broker” called SafeGraph initially provided the data to the CDC for free during the outbreak of the pandemic, the documents show. Then in 2021, the CDC reportedly hatched a deal to pay the company $420,000 for continued access.
Read the original article by clicking here.

Luke 21:11 There will be great earthquakes, and in various places famines and pestilences. And there will be terrors and great signs from heaven.
Important Takeaways:
- Malaria, a potentially deadly disease caused by a mosquito-borne parasite, is making inroads into the US.
- Five new cases of malaria — one in Texas and four in Florida — are alarming officials because they were locally acquired, meaning a mosquito in the US was carrying the parasite.
- That hasn’t happened since 2003 in Palm Beach County, Florida, according to the Centers for Disease and Prevention.
- Almost all cases of malaria now seen in the US are from people who traveled outside the country, where they were exposed to disease-carrying mosquitoes.
- But these five new cases — seen in people who hadn’t traveled abroad — raise fears that local mosquitoes could be spreading the disease to other people.
- But people with the parasite in their blood don’t always have symptoms, making it easy for the disease to spread when an asymptomatic person is bit.
- Symptoms of malaria include fever, shaking, chills, headache, muscle aches, nausea, vomiting, diarrhea and tiredness, according to the CDC.
- If it’s not treated promptly, the infection can cause jaundice, anemia, kidney failure, seizures, mental confusion, coma and death.
Read the original article by clicking here.

Luke 21:11 There will be great earthquakes, and in various places famines and pestilences. And there will be terrors and great signs from heaven.
Important Takeaways:
- CDC confirms heart disease risk soars 13,200 percent among vaccinated
- A bombshell study from the U.S. Centers for Disease Control (CDC) and the Food and Drug Administration (FDA) has confirmed that the risk of autoimmune heart disease is 13,200% higher in people who are vaccinated for Covid.
- The study found that the risk of myocarditis following mRNA Covid vaccination is around 133x greater than the background risk in the population.
- The study’s authors used data obtained from the CDC’s VAERS reporting system.
- The data was then cross-checked to ensure the results complied with the CDC’s definition of myocarditis.
- The researchers also noted that given the passive nature of the VAERS system, the number of reported incidents is likely to be an underestimate of the extent of the phenomenon.
- 1626 cases of myocarditis were studied.
- The results showed that the Pfizer-BioNTech product was most associated with higher risk.
- The Pfizer jabs caused 105.9 cases per million doses after the second vaccine shot in the 16 to 17 age group for males.
- In the 12 to 15 age group for males, 70.7 cases per million doses were recorded after the second shot.
- The 18 to 24 male age group also saw significantly higher rates of myocarditis for both Pfizer’s (52.4 cases per million) and Moderna’s (56.3 cases per million) products.
- Researchers also noted that 82 percent of cases were in males, consistent with previous studies.
- The risk was highest after the second vaccination dose in adolescent males and young men.
Read the original article by clicking here.












