
Important Takeaways:
- Meta CEO Mark Zuckerberg made weighty promises to Congress on Monday ahead of the 2024 election, saying he planned to fight any pressure from the White House to censor content on his social media platform and that he would not donate his controversial “Zuckerbucks” in this year’s election.
- Zuckerberg said in his most explicit criticisms yet of the Biden White House that senior officials “repeatedly pressured” the tech billionaire’s company to suppress content related to COVID-19 on its Facebook platform in 2021.
Read the original article by clicking here.

Important Takeaways:
- The group of medical experts banded together to launch research studies and share data after concluding there was compelling evidence among their own patients to suggest a link between COVID and cancer diagnoses
- “I’ve been in practice 23 years and have never seen anything like this,” Kashyap Patel, an oncologist in South Carolina and CEO of Carolina Blood and Cancer Care Associates
- Patel, who is calling for a national registry to analyze trends, said he has already collected data from dozens of his own patients showing a possible link between unusual cancers and long COVID.
- “Hopefully, we’re wrong,” Afshin Beheshti, president of the COVID-19 International Research Team, said. “But everything is, unfortunately, pushing toward that being the case.”
- The US-based doctors are calling on the federal government to prioritize the research — given such answers could affect treatment for cancer patients, as well as management of the disease, over the next several decades.
Read the original article by clicking here.

Luke 21:11 There will be great earthquakes, and in various places famines and pestilences. And there will be terrors and great signs from heaven.
Important Takeaways:
- The Great COVID Grift: $420 BILLION lost from coronavirus aid through ‘fraud and waste’: Staggering report reveals how billions of taxpayer cash from $4.2 trillion in bailout funds disappeared
- More than $400 billion of COVID aid was lost to fraud or wasted spending under Donald Trump and Joe Biden, a new bombshell report claimed on Monday.
- An analysis by the Associated Press said fraudsters and criminals plundered billions of taxpayer cash set aside to fend off economic collapse during the pandemic.
- It estimated that $280 billion in COVID relief funding was lost to fraud, while another $123 billion was wasted or misspent.
- Michael Horowitz, the U.S. Justice Department inspector general investigating Covid-related fraud, recently told Congress that it was ‘clearly in the tens of billions of dollars and may eventually exceed $100 billion.
Read the original article by clicking here.

Proverbs 29:2 When the righteous thrive, the people rejoice; when the wicked rule, the people groan.
Important Takeaways:
- Universal flu vaccine based on mRNA tech to be tested by National Institutes of Health
- NIH is enrolling patients in an early-stage clinical trial to test a universal flu vaccine based on mRNA technology.
- The technology is behind Moderna’s and Pfizer’s widely used Covid vaccines.
- Scientists hope the vaccine will protect against a wide variety of flu strains and provide long-term immunity so people do not have to receive a shot every year.
- The study will also include participants who receive a quadrivalent flu vaccine, which protects against four strains of the virus, to compare the experimental universal shot to those currently on the market.
Read the original article by clicking here.

Luke 21:11 There will be great earthquakes, and in various places famines and pestilences. And there will be terrors and great signs from heaven.
Important Takeaways:
- Chinese lab leak likely behind COVID outbreak: US Energy Department
- The virus that causes COVID-19 most likely leaked from a Chinese laboratory, according to a classified report based on new intelligence and recently sent to the White House and key members of Congress.
- The stunning assertion by the US Energy Department comes more than a year after the FBI concluded a lab accident in China was the origin of the disease, which has killed more than 6.8 million people around the world, including 1.1 million in the US.
- The FBI’s decision was made with “moderate confidence” and remains the bureau’s opinion, said The Wall Street Journal, which first reported the Energy Department’s own finding on Sunday.
- By contrast, the Department of Energy made its determination with “low confidence,” sources who’ve read the classified report told the Journal.
- The Energy Department declined to discuss details of its assessment but said in a statement that it “continues to support the thorough, careful, and objective work of our intelligence professionals in investigating the origins of COVID-19, as the President directed.”
- Biden ordered US spy agencies to probe the source of the disease — including whether it came from a Chinese lab in Wuhan– in May 2021, more than a year after the World Health Organization declared COVID-19 a global pandemic.
Read the original article by clicking here.

Matthew 24:12 And because lawlessness will be increased, the love of many will grow cold
Important Takeaways:
- Violence hits ‘epidemic proportions’ in pandemic-era California, study shows
- According to the new annual report from the California Study on Violence Experiences across the Lifespan (CalVEX), violence statistics have seen a significant increase since COVID-19 emerged. The report, conducted by scientists at the University of California San Diego School of Medicine, reports more than one in six Californians (18%) experienced either physical or sexual violence in just the past year. Meanwhile, one in every 25 Californians experienced intimate partner violence.
- Physical violence nearly doubling among men between 2020-2022. Study authors say demographic disparities in the results may provide further insight into potential contributing factors that could have been exacerbated during the pandemic.
- A professor at University of California San Diego School of Medicine and Division of Social Sciences, in a press release. “Current violence prevention efforts are clearly woefully inadequate…
Read the original article by clicking here.
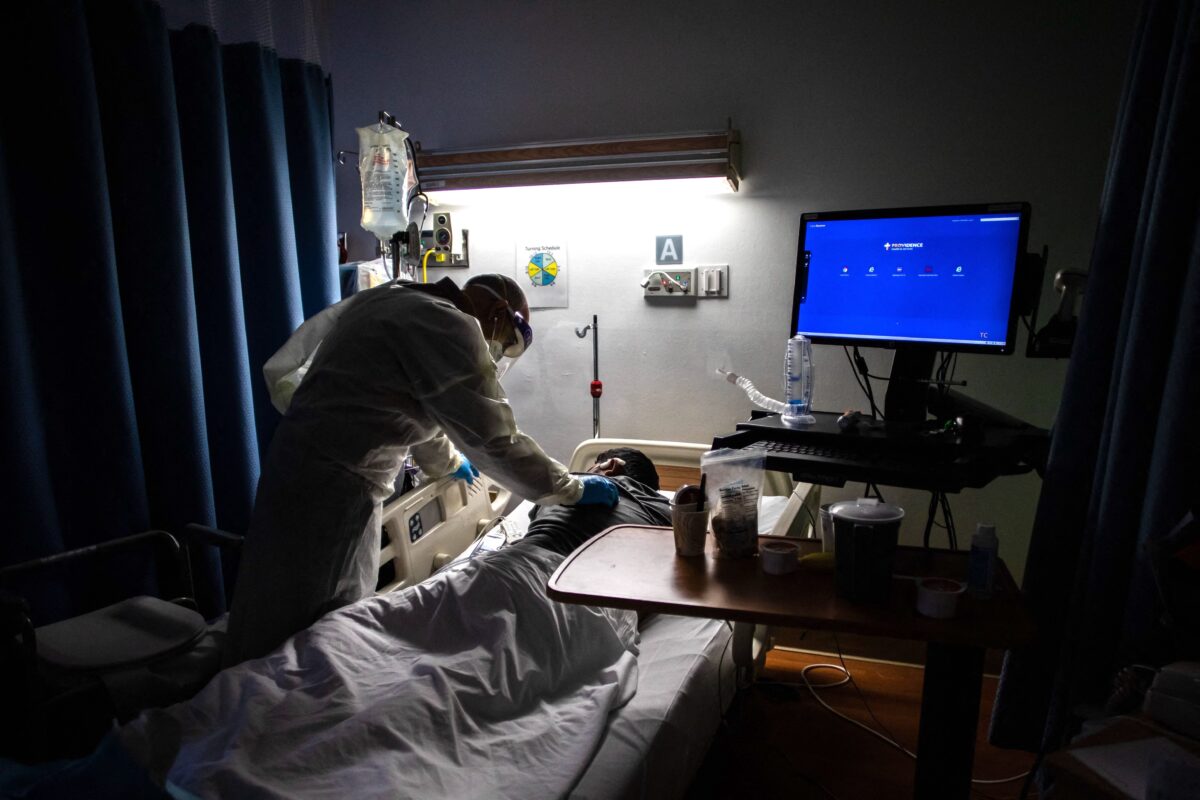
- California Lawmakers Pass Bill to Punish Dissenting Doctors for ‘Misinformation’
- Legislation that would punish doctors who dissent from the California government’s messaging on COVID-19 has passed the state Legislature
- The final amendments to Assembly Bill 2098 (AB 2098), introduced by Assemblyman Evan Low (D-Campbell), were passed by a 56-20 vote on Aug. 30 in the Assembly.
- The bill would amend the state’s Business and Professions Code to give the Medical Board of California (MBC) the discretionary power to discipline physicians or surgeons who spread “misinformation or disinformation” related to COVID-19. The MBC currently has the power to punish doctors charged with unprofessional conduct under the Medical Practice Act for violations including gross negligence, incompetence, dishonesty, or corruption.
- “Misinformation,” according to the Aug. 23 Senate Floor analysis, means “false or misleading information about the nature and risks of the virus; COVID-19 prevention and treatment; and the development, safety, and effectiveness of COVID-19 vaccines.”
- Michael Huang of Roseville, Calif., who is in jeopardy of losing his medical license for allegedly issuing invalid mask and vaccine medical exemptions to his patients (many of them firefighters and school-age children)
- “It’s horrible. We’re back into the days of the Inquisition when Galileo was found guilty for saying that the Earth revolves around the sun,” he said. “The state is acting well beyond what it’s designed to do.”
Read the original article by clicking here.

Matt 24:7 For nation will rise against nation, and kingdom against kingdom. And there will be famines, pestilences, and earthquakes in various places.
Important Takeaways:
- Daily reported cases have exceeded the peak of last summer’s delta wave
- Oakland International Airport tweeted that everyone over the age of 2 must once again wear masks in indoor settings there
- Masks will be required in all other children and youth settings, including summer school and youth programs.
- AC Transit also announced that it would restore its mask mandate policy on all buses “until further notice.”
Read the original article by clicking here.

Revelations 6:3-4 “when he opened the second seal, I heard the second living creature say, “Come!” 4 And out came another horse, bright red. Its rider was permitted to take peace from the earth, so that people should slay one another, and he was given a great sword.
Important Takeaways:
- North Korea fires 3 ballistic missiles amid 1st virus outbreak
- North Korea fired three short-range ballistic missiles toward the sea on Thursday, South Korea’s military said, in the latest of a series of weapons demonstrations this year that came just hours after it confirmed its first case of the coronavirus since the pandemic began.
- South Korea’s military has boosted its readiness and surveillance while maintaining close coordination with the United States.
- Earlier Thursday, North Korean state media confirmed the country’s first COVID-19 infections as Kim ordered nationwide lockdowns to slow the spread of the virus. Kim also ordered officials to bolster the country’s defense posture to avoid any security vacuum.
Read the original article by clicking here.












